38 eu language requirements for product labels
EU Labeling Requirements - United States Mission to the European Union Nutrition labeling becomes mandatory on December 13, 2016 Minimum font size for printing mandatory information New format for allergen labeling (allergens must be highlighted in the list of ingredients - "allergen boxes" are no longer allowed) Voluntary front-of-pack labeling has to follow a set format sewport.com › learn › garment-labeling-and-requirementsGarment Labelling Requirements for Clothing (Full Guide) Jan 13, 2019 · Labeling Regulations and Requirements in the EU. The EU has laid down a variety of regulations regarding the labeling of textile products that are sold within its member nations. These textile labels must be provided in the native language of the member state where the garments are sold, and they must also include the following information: 1.
EU - Labelling Requirements | CE Intelligence The use of language on labels has been the subject of a Commission Communication, which points out that labelling of foodstuffs for sale to the final consumer must be in an easily understandable language which is generally interpreted to mean the language of the country of marketing (European Commission ,2010).

Eu language requirements for product labels
Access2Markets EU-Vietnam Free Trade Agreement - Europa Aug 01, 2020 · The agreement at a glance. The EU-Vietnam Free Trade Agreement entered into force on 1 August 2020.. The EU-Vietnam Investment Protection Agreement will enter into force after all EU Member States have given it their formal consent.. The trade agreement. removes customs duties, red tape and other obstacles that European firms face when exporting to … EU Language Requirements | Obelis The table below provides an overview of the official languages in the EU Member States with the aim to assist the manufacturer to know which languages should appear on the labels of cosmetic products to be place on specific markets. Product Labeling Requirements: What You Need To Know The US, Canada, Mexico, and the EU all require that your product packaging be written in local languages. In Canada, that means your labels need to be in English and French. In Mexico, that means Spanish. Choosing a Labeling Translation and Compliance Partner
Eu language requirements for product labels. Identifying product requirements - Your Europe In particular, the requirements under national rules might differ for the: size/dimensions weight composition labelling packaging testing To find out which technical rules apply to specific products in each EU country or the details of competent authorities within that EU country you can contact the Product Contact Points. EU GMP Requirements - European Medicines Agency Data complete, according to CTA, right language (Core and translated) label text approved Printing process, e.g.: - each printing run and collection of printed labels separately - measures to avoid misprinting - reconciliation of amounts - change of use-by date: usually at authorised site, no superimposing batch ID. Control of printed labels europa.eu › youreurope › businessEcodesign requirements in the EU - Your Europe Apr 11, 2021 · Specific requirements. Specific requirements are when exact values are measured and a limit is given. For example, maximum energy consumption, or minimum quantities of recycled material to be used in production. Generic requirements. Generic requirements do not set limit values, but may require that: the product is 'energy-efficient' or ... European Union - Labeling/Marking Requirements (part 1) | export.gov MANDATORY MARKS AND LABELS CE Marking This is probably the most widely used and recognized marking required by the EU. Found in all "New Approach" legislation with a few exceptions, the CE marking demonstrates that a product meets all essential requirements (typically related to safety, health, energy efficiency and/or environmental concerns).
EU MDR language requirements - Decomplix A glance at the MDR shows that Article 10 (11) very fundamentally requires manufacturers to include information in one or more official language (s) determined by the Member State in which the product is made available. In addition to labelling requirements, it also requires that this information be clearly understandable to the intended users ... A Brief Reminder of the Language Requirements - Biorius BIORIUS realized that many of its clients are confused by the language requirements in the EU. Although this aspect falls under the responsibility of your distributors (according to Article 6 of the EU Cosmetics Regulation), it is important to make a reminder of the rules currently in application as the design and edition of labels and packaging is a costly exercise for cosmetic brand owners. PDF WHAT YOU NEED TO KNOW MDR LANGUAGES & LABELING - Network Partners The European Union Medical Device Regulation (MDR 2017-745) includes new requirements for labeling and languages. Labeling is a critical element of a company's medical device offerings and should be provided in the language appropriate for end users. Package or product labels shall include translations of the product How to Create a Label as per EU MDR 2017/745? Innovations by MDR 2017/745. Manufacturers of medical devices are subject to a number of new requirements as a result of the EU Medical Device Directive, mainly the following: The scope of application also extends to non-medical products (e.g. contact lenses, devices for liposuction). Each medical device must bear a unique identification number ...
Country of Origin Requirements in the United States: An Overview Apr 27, 2022 · If a product is fully manufactured in a single country, then determine the Country of Origin is straightforward. However, the decision might be more complex, if a product is processed in more than a country, or if it processed in a country using components imported from another country – which is often the case. Examples What, if any, are the EU Requirements for Minimum Font Size for a ... At the Community level, there is no general rule which requires a minimum font size for warning labels for non-food consumer packaged products. However, certain products can be subject to specific requirements imposed by Community legislation or, in the absence of EC law, to specific rules imposed by the national laws of the Member States where the product is marketed. Medical Devices Labeling Checklist for EU MDR Compliance Medical Device Labeling Requirements as per EU MDR: If there is no expiration date, manufacturers should include the device's name and trade name, as well as the manufacturing date. A Standardized Symbol/Logo/ ICON must appear on all labels of the Product, indicating that the product being delivered into the Europe Union includes a medical ... Labelling and packaging - ECHA The label should be firmly attached to one or more of the packaging's surfaces and has to include the following: The name, address and telephone number of the supplier The nominal quantity of a substance or mixture in packages made available to the general public (unless this quantity is specified elsewhere on the package) Product identifiers
europa.eu › youreurope › businessCE marking – obtaining the certificate, EU requirements ... Mar 26, 2021 · CE marking is only obligatory for products for which EU specifications exist and require the affixing of CE marking. Some products are subject to several EU requirements at the same time. You must make sure that your product complies with all the relevant requirements before affixing the CE marking to it.
European Union Product Labeling Requirements: A Complete Guide Below follows criteria for Textiles and Furniture: Textiles 1. The product shall not contain lead-based pigments. 2. Manufacturers shall perform colorfastness, washing, wet rubbing, dry rubbing tests on dyed yarn, final fabrics, or final products. 3. Manufactured elastane shall not contain organotin compounds Furniture 1.
EU MDR – Medical Device Labeling Changes & Challenges Sep 08, 2021 · Global rollout of EU MDR and other UDI-type of regulations are driving all medical device companies to revisit their labeling processes to ensure they are all compliant across the extended supply chain. The European Medical Device Regulations (MDR) 2017/745 and In Vitro Diagnostic Regulations (IVD) 2017/746 were published on May 5, 2017 in the Official […]
Access2Markets Labelling and packaging - Europa The information provided by labels must be easy to understand, easily visible, clearly legible and indelible and must appear in the official language (s) of the Member State where the product is marketed. However, the use of foreign terms or expressions easily understood by the purchaser may be allowed. List of applicable legislation
Product Labelling — EUbusiness.com | EU news, business and politics Almost all products sold within the EU will require some form of labeling, but what details are required depends on the nature of the product. Here are a few examples of the topics covered by EU legislation: EU Food Product Labeling; General legislation covering labeling, presentation and advertising Nutritional Alcoholic beverages
Product Labeling Regulations in the US, EU and Australia Warning labels and user instructions. Some labeling requirements apply to all, or a wide range of, product categories. For example, all products in the US must be labelled with the country of origin (i.e., Made in China). In the European Union, many products must be CE marked. Other labeling requirements apply to specific products.
Nutrition declaration - EU labelling rules - Your Europe Jun 12, 2021 · If the product requires preparation before it is consumed, you can provide the information that reflects the nutrition values of the food as ready to be eaten. The energy value must be: calculated using the conversion factors provided in annex 14 of the regulation
EU MDR Label Translation Requirements - Supplier Help Center Question: What are the translation requirements for product labels under the European Union (EU) Medical Device Regulation (MDR)?Do labels need to be translated into the languages of EU member states where products are marketed? Answer: MedTech Europe, the European trade association for the medical technology industry, has published a guidance document on symbol usage for medical device labels.
EU - Labeling/Marking Requirements Starting on July 16, 2021, all CE marked products will need to have an EU address on the label. This also applies to products sold online. The name and address must appear on the product or the product's packaging so that customs and market surveillance authorities can have a contact person in case the product is suspected to present a risk.
CE marking – obtaining the certificate, EU requirements Mar 26, 2021 · CE marking is only obligatory for products for which EU specifications exist and require the affixing of CE marking. Some products are subject to several EU requirements at the same time. You must make sure that your product complies with all the relevant requirements before affixing the CE marking to it.
European language labeling for Medical Devices CE Mark EU Foreign Language Labeling Requirements There are two major areas of confusion about the translation requirements when CE marking a product for export to the EU States. One is that everyone talks about "CE Marking translation requirements" without actually reading the specific Directive (s) that apply to their products.
Labels and markings - Your Europe Mandatory labels Many products must bear the CE marking before they can be sold in the EU - no matter where they were manufactured. Check when the CE marking is mandatory, whether it applies to your products and how to affix it. CE marking If you intend to sell electrical appliances, find out what your responsibilities are and what you need to do.
Garment Labelling Requirements for Clothing (Full Guide) Jan 13, 2019 · Labeling Regulations and Requirements in the EU. The EU has laid down a variety of regulations regarding the labeling of textile products that are sold within its member nations. These textile labels must be provided in the native language of the member state where the garments are sold, and they must also include the following information: 1.
Sell on Amazon in Europe | Amazon Global Selling There are many other marks and labels in Europe (for example, textiles, products in contact with food, recycling, and so on), which you may be required to display on your products or packaging. Often product labeling is required to be in the language of the European member state where the product is sold.
Product-information requirements | European Medicines Agency EMA's guidance explains the content that should be included in these documents, as well as standard headings and the most commonly used standard statements and terms in all official European Union (EU) languages plus Icelandic and Norwegian, and defines the format and layout for the product information. EMA's guidance is without prejudice to:


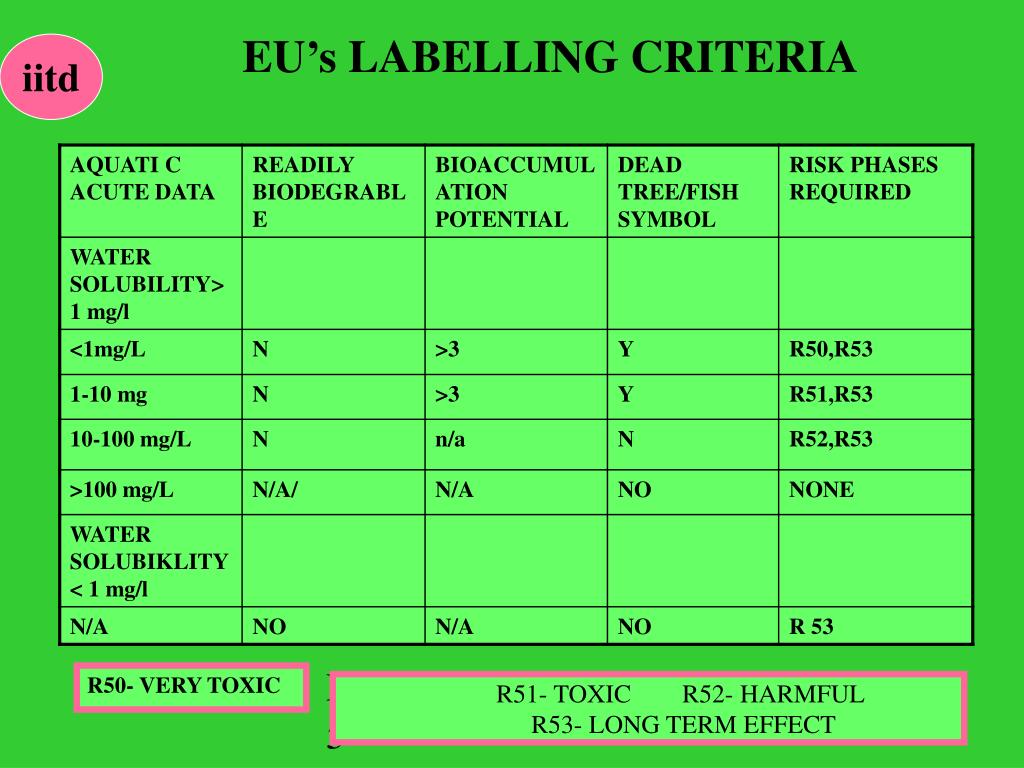









Post a Comment for "38 eu language requirements for product labels"