45 fda relaxing food labels
Food Allergen Labeling—What Happens Next? - Celiac.com Food labels must list—in everyday language—any of the eight main food allergens ( milk, egg, peanuts, tree nuts, fish, shellfish, soy, and wheat) that are in the product. Allergens in flavoring, coloring, or incidental additives to be labeled in accordance with these requirements. Food Labels Revealed - Podbean Since this episode comes at the end of 2021, I decided to put together a retrospective program which has highlights from the first 20 episodes, including such topics as GRAS, nutrition facts labels, Lunchables, gum, ice cream, and bread.
› cms_ia › importalert_190Import Alert 66-41 - Food and Drug Administration If a firm and/or a representative thereof would like to petition for removal from detention without physical examination under this Import Alert, all relevant information supporting the request should be forwarded to the following address: Food and Drug Administration Division of Import Operations 12420 Parklawn Drive, ELEM-3109 Rockville, MD ...

Fda relaxing food labels
The New Nutrition Facts Label | FDA - U.S. Food and Drug Administration The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific ... CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) (i) Except as provided in paragraph (a) (1) (ii) of this section, the applicant must notify FDA about each change in each condition established in an approved NDA beyond the variations already... Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
Fda relaxing food labels. › food › current-good-manufacturingGood Manufacturing Practices for the 21st Century for Food ... In an FDA survey of 85 small, medium, and large food plants, FDA and state inspectors found that only half of the firms were cross-checking ingredients on the labels with ingredients used in ... The FDA Finally Liberates French Dressing from 72-Year-Old Ingredient ... The FDA agrees with this observation, seeing "a proliferation of nonstandardized pourable dressings for salads with respect to flavors (Italian, Ranch, cheese, fruit, peppercorn, varied vinegars,... FDA Warns 4 Products For Selling Honey Products With Viagra - Fatherly July 14, 2022. Jason Armond/Los Angeles Times/Getty Images. The U.S. Food and Drug Administration (FDA) issued a warning to four companies after discovering they sold products with ingredients that may "pose a significant health risk to consumers." The companies sell honey-based products marketed to people as " sexual enhancement remedies ... FDA Will Ease Enforcement of Baby Formula Regulations To Address Shortage On Monday, the FDA announced that it would temporarily ease enforcement of some labeling rules in order to allow for the importation of foreign formulas that meet U.S. safety and nutrition...
Organic on Food Labels | FDA For more information on the use of the term "organic" on food labels and USDA requirements, go to the National Organic Program website. Content current as of: 03/07/2022 Import Alert 80-06 - Food and Drug Administration ***The Food and Drug Administration has warned and/or taken action against a number of companies that have made improper claims about their products' intended use. Some may carry significant health risks. Section 502(a) declares that a drug or device is misbranded if its labeling proves false or misleading in any particular. Compliance & Enforcement (Food) | FDA To protect public health, FDA monitors domestic firms and the foods that they produce. FDA also has multiple initiatives for monitoring imported products and foreign firms exporting to the United... Structure/Function Claims | FDA - U.S. Food and Drug Administration If a dietary supplement label includes such a claim, it must state in a "disclaimer" that FDA has not evaluated the claim. The disclaimer must also state that the dietary supplement product is not...
How Food Processors Are Working to Avoid Allergens Until last year, the FDA had called out eight foods as "major" allergens: Peanuts Tree nuts Fish Shellfish Wheat Eggs Milk Soy But with the 2021 passage of the Food Allergy Safety, Treatment, Education and Research (FASTER) Act, sesame has been added to the list; enforcement will begin next year. Ex-FDA official reveals how agency caused baby-formula crisis Manhattan mom Amy Daly, 38, of the Upper West Side lamented Friday that she was forced to take her 11-month-old baby Alice off infant formula early when the shortage hit. Former FDA associate ... FDA to ease baby formula import rules as Biden faces heat - New York Post A loosening of import restrictions could have a significant impact. Currently, about 98% of US-consumed formula is produced domestically. The FDA announced it will relax import rules on baby... › drugs › 2Myrbetriq Oral: Uses, Side Effects, Interactions ... - WebMD Mirabegron works by relaxing a certain ... Children should take this medication with food. Swallow this medication whole. ... You may report side effects to FDA at 1-800-FDA-1088 or at ...
› foodSan Francisco Restaurant Reviews, Recipes, Wine & Spirits ... Find food and wine reviews and news on San Francisco restaurants, recipes, cooking, chefs, cocktails and bars — SFGate
Food Safety Priorities for 2022 - Food Processing The FDA has already penciled in the addition of sesame to the list of allergens that must be declared on food labels, although enforcement doesn't begin till Jan. 1, 2023. That brings the total number of allergens to nine, the other eight being eggs, tree nuts, peanuts, cow's milk, fish, shellfish, soy and wheat.
Daxxify: 8 Things to Know About the New Botox Alternative The U.S. Food and Drug Administration (FDA) has approved Daxxify, a new injected medicine for smoothing facial wrinkles in adults, which dermatologists see as the first major competitor to Botox ...
FDA Bans JUUL Products From US Market - Cannabis Business Times The U.S. Food and Drug Administration (FDA) has issued marketing denial orders (MDOs) to JUUL Labs Inc., effectively banning the company's products from the U.S. market. "As a result, the company must stop selling and distributing these products," FDA officials said in a press release issued June 23. "In addition, those currently on the U.S. market must be removed, or risk enforcement ...
Food Allergen Labeling and Consumer Protection Act of 2004 SEC. 203. FOOD LABELING; REQUIREMENT OF INFORMATION REGARDING ALLERGENIC SUBSTANCES. (a) In General.--Section 403 of the Federal Food, Drug, and Cosmetic Act (21 U.S.C. 343) is amended by adding ...
What Information Should Be on Drug Labels? - MedicineNet Here is what is usually found on a label: Classification The product and its use are described on the front and side panels of a drug container. There will be some indication of the product's classification next to the name. The Pr symbol indicates that the item is a prescription drug. No Pr denotes that no prescription is required to purchase it.
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Dried yeast ( Saccharomyces cerevisiae and Saccharomyces fragilis ) and dried torula yeast ( Candida utilis ) may be safely used in food provided the total folic acid content of the yeast does not exceed 0.04 milligram per gram of yeast (approximately 0.008 milligram of pteroyglutamic acid per gram of yeast).
FDA Releases New Salt Guidelines: How to Slash Your Sodium Intake Here's how the FDA recommends reading the labels: The Daily value (DV) for sodium is less than 2,300 milligrams (mg) per day. The percent DV shows how much of the maximum recommended amount of...
Bubs baby formula from Australia gets FDA import approval The Food and Drug Administration gave the green light to an Australian company to import its baby formula after relaxing strict regulatory requirements aimed at alleviating a critical domestic shortage. ... The FDA is exercising enforcement discretion for certain infant formula products after determining that microbiological testing, labeling ...
Daxxify, an FDA-Approved Wrinkle Treatment, Is Longer Lasting Than Botox The Food and Drug Administration (FDA) recently approved Daxxify, a new anti-wrinkle treatment that's similar to Botox, but its effects can last much longer. Made by Revance Therapeutics, Daxxify is a neuromuscular blocking agent that "paralyzes" wrinkles the same way Botox does.
greenroads.com › collections › cbd-edibles-gummiesBuy CBD Gummies & CBD Edibles Online | Green Roads The FDA forbids all CBD companies from publishing reviews in which customers mention specific health conditions. As you might imagine, a large number of our consumer reviews mention specific health conditions. In order to comply with the FDA, we’ve published a representative selection of reviews that pass the FDA guidelines.
Changes to the Nutrition Facts Label | FDA - U.S. Food and Drug ... The updated label appears on the majority of food packages. Manufacturers with $10 million or more in annual sales were required to update their labels by January 1, 2020; manufacturers with less ...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration Subpart A - General Provisions. Sec. 310.3 Definitions and interpretations. (a) The term act means the Federal Food, Drug, and Cosmetic Act, as amended (secs. 201-902, 52 Stat. 1040 et seq., as amended; 21 U.S.C. 321-392). (b) Department means the Department of Health and Human Services. (c) Secretary means the Secretary of Health and Human ...
› MagniLife-RLJ-114-MagnilifeAmazon.com: Magnilife Relaxing Leg Cream : Health & Household This item Magnilife Relaxing Leg Cream Dr. Bronner's - Organic Magic Balm (Arnica-Menthol, 2 Ounce) - Made with Organic Beeswax and Organic Hemp Oil, Relieves and Relaxes Sore Muscles and Achy Joints, Moisturizes and Soothes Dry Skin
Machine learning-based quantitative prediction of drug ... - Nature Construction of the PK-DDI DB. A total of 38,711 FDA drug label data for 3,587 prescription drugs was downloaded in an XML format from the DailyMed website. After applying standard operating ...
Food Labeling & Nutrition | FDA Food labeling is required for most prepared foods, such as breads, cereals, canned and frozen foods, snacks, desserts, drinks, etc. Nutrition labeling for raw produce (fruits and vegetables) and ...
› drugs › 2WebMD - Better information. Better health. WebMD - Better information. Better health.
Label Claims for Food & Dietary Supplements | FDA Among the claims that can be used on food and dietary supplement labels are three categories of claims that are defined by statute and/or FDA regulations: health claims, nutrient content claims,...
CFR - Code of Federal Regulations Title 21 - Food and Drug Administration (1) (i) Except as provided in paragraph (a) (1) (ii) of this section, the applicant must notify FDA about each change in each condition established in an approved NDA beyond the variations already...
The New Nutrition Facts Label | FDA - U.S. Food and Drug Administration The U.S. Food and Drug Administration (FDA) has updated the Nutrition Facts label on packaged foods and drinks. FDA is requiring changes to the Nutrition Facts label based on updated scientific ...
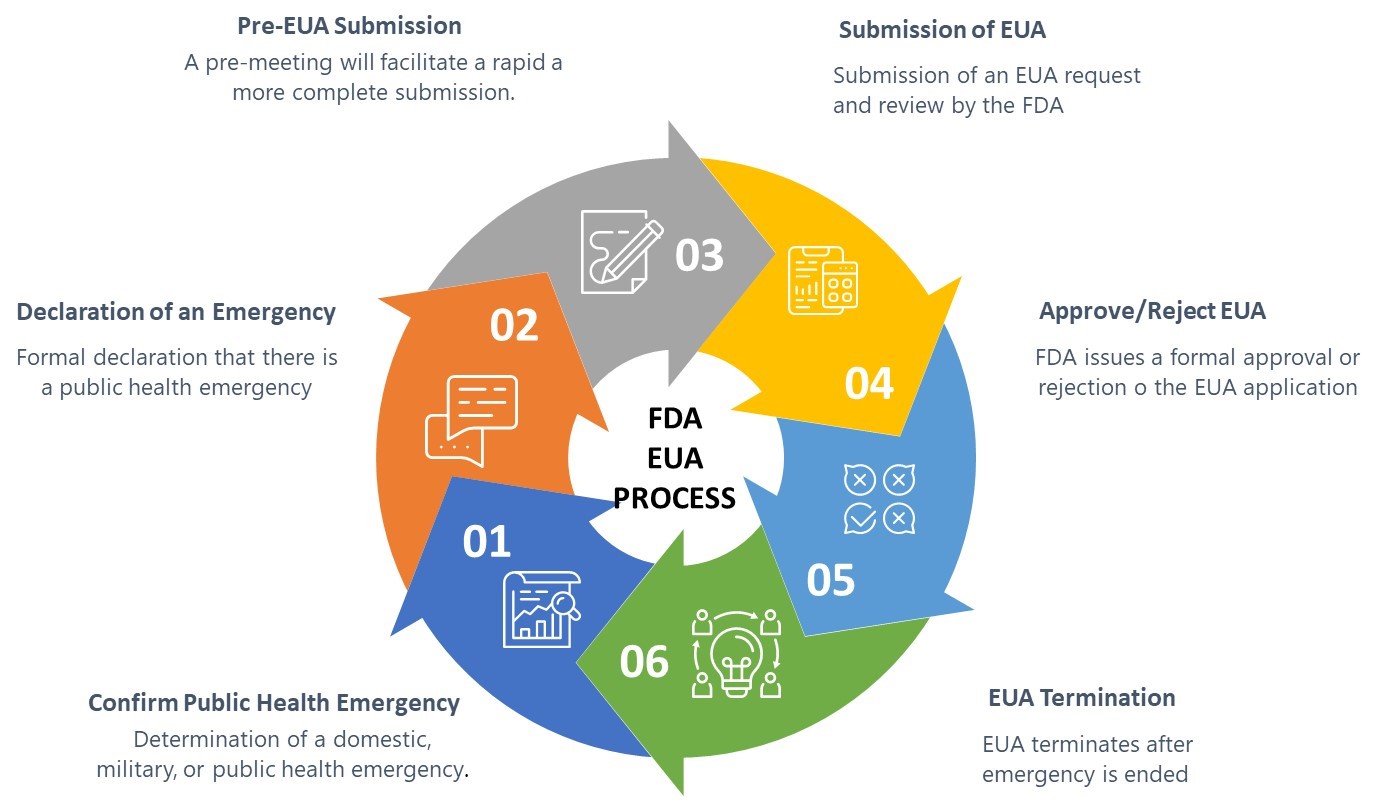




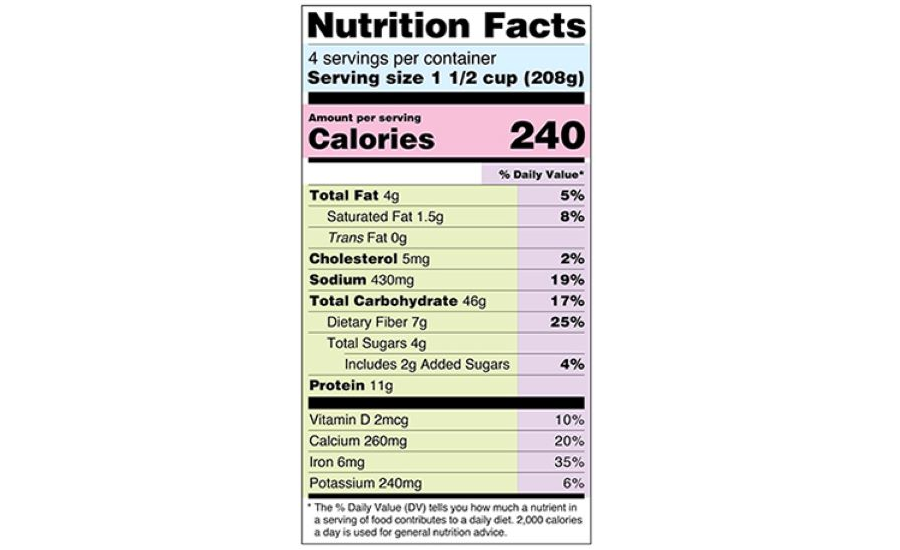



















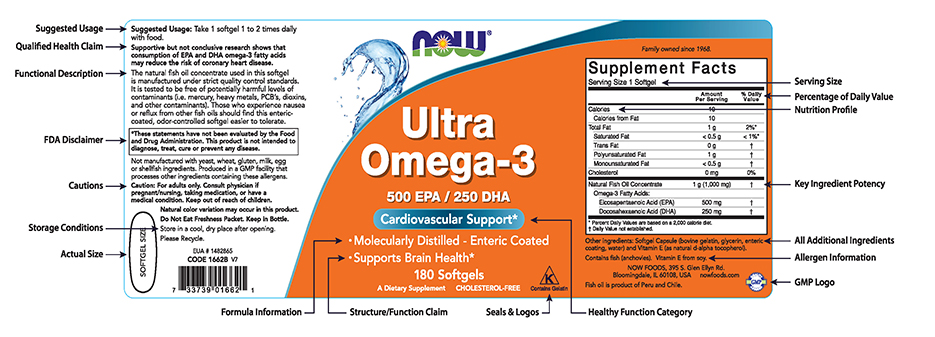











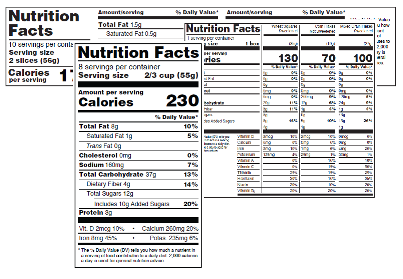
Post a Comment for "45 fda relaxing food labels"